How do buffer solutions work. Buffer to follow.

Acid Base Equilibria Buffer Solution Youtube
Its actually somewhere in the middle.
. Buffering ensures that videos can play smoothly and continuously. How does the buffer system work. Its 9 frames to buffer. With triple buffering off and vysnc on it drops to 30 fps.
And by the way D3DOverrider forces triple buffering by hooking the application and modifying the Direct3D back buffer count during swap chain creation giving you an audible tone during success or failure. Buffering is a technique to storing a data before it is work. Its like OP said. I believe that Google havet made it open source or even public for that matter Yes Google backs the largest mobile OS which is free but they are not going to give out their proprietary algorithms to the public if that was the case then.
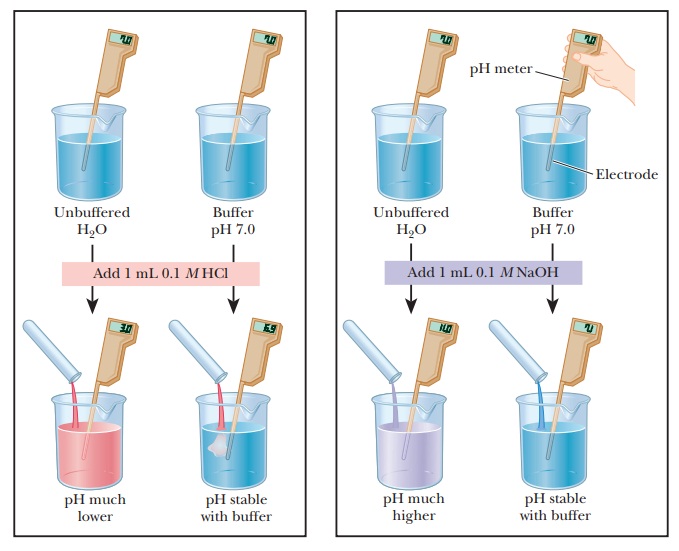
What is a buffer and how does a buffer work. Lets take an example of a buffer made up of the weak base ammonia NH3 and its conjugate acid NH4. Buffers work by neutralizing any added acid H ions or base OH- ions to maintain the moderate pH making them a weaker acid or base. A buffer is simply a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid.
When my draw time is greater than 160th of a second my frame rate does not drop to 30 fps with triple buffering on. The more H you add the more dissociation of water occurs moving the equation to the right. When HCl strong acid is added to this buffer system the extra H ions added to the system are consumed. When you burn your CD or printing your file then computer first store data in temporaryrandom memory ie.
Acidic and alkaline buffer solutions achieve this in different ways. When an acid soil is mixed with the buffer solution the pH of the soil. What is a buffer and how does a buffer work. How does a mixture of a weak acid and its conjugate base help buffer a solution against pH changes.
Click to see full answer Thereof how does the bicarbonate buffer system work. How does the bicarbonate buffer system work. This is known as buffering. If we mix a weak acid HA with its conjugate base A- both the acid and base components remain present in the solutionThis is because they do not undergo any reactions that significantly alter their concentrations.
Buffers work by neutralizing any added acid H ions or base OH- ions to maintain the moderate pH making them a weaker acid or base. They say 10 because 9 frame buffer window 1 first actionable frame 10 frames to place an input for the action to come out as early as possible. It works more or less like this. Its a weak base and its conjugated acid that make up the buffer.
It comes with three settings. Lets take an example of a buffer made up of the weak base ammonia NH3 and its conjugate acid NH4. Network Buffering can smoothen the gameplay experience for players who constantly face packet losses. Buffer mixture increases above that of the soil alone but does not.
As a weak base Tris is in an equilibrium with its strong conjugated acid. If you were to add more H to this solution more H 2 O would dissociate to generate a matching amount of OH. For example in brawl you used to be able to buffer multiple inputs allowing BDACUS. A buffer solution is a combination of chemicals that are formulated to change pH slowly with increased acidity.
In red blood cells carbonic anhydrase forces the dissociation of the acid rendering the blood less acidic. Tris has a pKa value of about 8 so it buffers between 7 and 9. Streaming media players load a few seconds of the stream ahead of time so that the video or audio can continue playing if the connection is briefly interrupted. Smash 4 the buffering has changed you cannot do multiple inputs.
Tris H_20 Tris-H OH -. Obviously triple buffering is doing something. Buffers work by reacting with any added acid or base to control the pH. The bicarbonate is regulated in the blood by sodium as are the phosphate ions.
A buffer solution has to contain things which will remove any hydrogen ions or hydroxide ions that you might add to it - otherwise the pH will change. Why does my streaming keep buffering If buffering is something that you are experiencing regularly it may be time to upgrade your internet speed. Minimum Moderate and Maximum. While it seems like your tracks are grayed out and cant be clicked many of the songs may still be cached and will play normally if you let it keep playing.
Now with a more complex scene I start to see benefits with triple buffering on. When sodium bicarbonate NaHCO 3 comes into contact with a strong acid such as HCl carbonic acid H 2. The bicarbonate-carbonic acid buffer works in a fashion similar to phosphate buffersThe bicarbonate is regulated in the blood by sodium as are the phosphate ions. Answer 1 of 2.
What you are experiencing is a magical thing called cache - files stored locally on the device. The bicarbonate-carbonic acid buffer works in a fashion similar to phosphate buffers. Spotify does not buffer 10 minutes ahead. As the above example shows a buffer works by replacing a strong acid or base with a weak one.
The pH will be slightly basic. Buffers work by neutralising any added acid H ions or base OH- ions to maintain the moderate pH making them a weaker acid or base. The buffering process may last just a few seconds for a shorter video or several minutes for a longer video. Well take a mixture of ethanoic acid and sodium ethanoate as typical.
Even when you have cell coverage you may still be. When HCl strong acid is added to this buffer system the extra H ions added to the system are consumed. A buffering action occurs here because water can dissociate into a strong acid and a strong base but balance each other out. Lets take an example of a buffer made up of the weak.

Buffer Solution What Is It And How Does It Work To Resist Changes In Its Ph

21 6 Buffer Solutions Flashcards Quizlet

What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio

Buffers Indicators Acids And Bases 101 The Basics Of Chemistry
